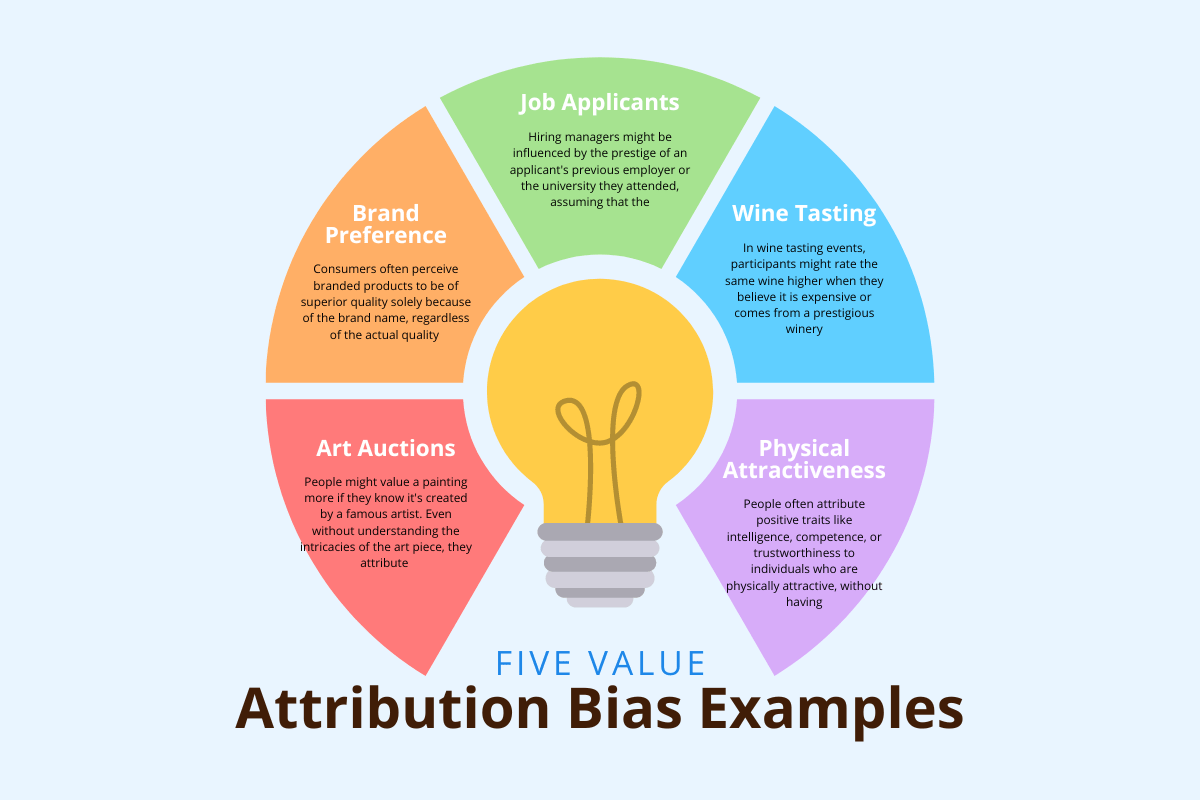
Indication Bias Definition . then, based on earlier work and current project activities, we developed a framework for the assessment of the risk of bias and confounding across a body of observational study. A distortion that modifies an association between an exposure and an outcome, caused by the presence of an indication for the exposure. confounding by indication is a bias frequently encountered in observational epidemiologic studies of drug effects. this jama guide to statistics and methods reviews the use of methods to control for confounding by indication in clinical studies that assess the potential effect of a. confounding by indication poses a significant threat to the validity of nonexperimental studies assessing effectiveness and safety. a type of confounding bias that occurs when a symptom or sign of disease is judged as an indication (or a contraindication) for a given.
from exoewimhv.blob.core.windows.net
confounding by indication is a bias frequently encountered in observational epidemiologic studies of drug effects. confounding by indication poses a significant threat to the validity of nonexperimental studies assessing effectiveness and safety. a type of confounding bias that occurs when a symptom or sign of disease is judged as an indication (or a contraindication) for a given. this jama guide to statistics and methods reviews the use of methods to control for confounding by indication in clinical studies that assess the potential effect of a. A distortion that modifies an association between an exposure and an outcome, caused by the presence of an indication for the exposure. then, based on earlier work and current project activities, we developed a framework for the assessment of the risk of bias and confounding across a body of observational study.
Examples Of Labeling Bias at Paul McKee blog
Indication Bias Definition confounding by indication is a bias frequently encountered in observational epidemiologic studies of drug effects. A distortion that modifies an association between an exposure and an outcome, caused by the presence of an indication for the exposure. confounding by indication poses a significant threat to the validity of nonexperimental studies assessing effectiveness and safety. this jama guide to statistics and methods reviews the use of methods to control for confounding by indication in clinical studies that assess the potential effect of a. confounding by indication is a bias frequently encountered in observational epidemiologic studies of drug effects. a type of confounding bias that occurs when a symptom or sign of disease is judged as an indication (or a contraindication) for a given. then, based on earlier work and current project activities, we developed a framework for the assessment of the risk of bias and confounding across a body of observational study.
From www.youtube.com
What is Attentional bias?, Explain Attentional bias, Define Attentional Indication Bias Definition this jama guide to statistics and methods reviews the use of methods to control for confounding by indication in clinical studies that assess the potential effect of a. then, based on earlier work and current project activities, we developed a framework for the assessment of the risk of bias and confounding across a body of observational study. . Indication Bias Definition.
From www.slideserve.com
PPT Bias in Clinical Research Measurement Bias PowerPoint Indication Bias Definition confounding by indication is a bias frequently encountered in observational epidemiologic studies of drug effects. A distortion that modifies an association between an exposure and an outcome, caused by the presence of an indication for the exposure. a type of confounding bias that occurs when a symptom or sign of disease is judged as an indication (or a. Indication Bias Definition.
From cemdaagm.blob.core.windows.net
What Does Social Bias Mean at Connie Morris blog Indication Bias Definition confounding by indication poses a significant threat to the validity of nonexperimental studies assessing effectiveness and safety. then, based on earlier work and current project activities, we developed a framework for the assessment of the risk of bias and confounding across a body of observational study. this jama guide to statistics and methods reviews the use of. Indication Bias Definition.
From www.youtube.com
What is Information Bias? [Definition and Example] Guide to Cognitive Indication Bias Definition a type of confounding bias that occurs when a symptom or sign of disease is judged as an indication (or a contraindication) for a given. A distortion that modifies an association between an exposure and an outcome, caused by the presence of an indication for the exposure. then, based on earlier work and current project activities, we developed. Indication Bias Definition.
From www.slideshare.net
Assessment of Bias Indication Bias Definition A distortion that modifies an association between an exposure and an outcome, caused by the presence of an indication for the exposure. confounding by indication is a bias frequently encountered in observational epidemiologic studies of drug effects. this jama guide to statistics and methods reviews the use of methods to control for confounding by indication in clinical studies. Indication Bias Definition.
From www.youtube.com
Research Bias 101 Selection Bias, Analysis Bias and Procedural Bias Indication Bias Definition confounding by indication is a bias frequently encountered in observational epidemiologic studies of drug effects. then, based on earlier work and current project activities, we developed a framework for the assessment of the risk of bias and confounding across a body of observational study. confounding by indication poses a significant threat to the validity of nonexperimental studies. Indication Bias Definition.
From www.marketing91.com
Confirmation Bias Definition, Types and Examples Marketing91 Indication Bias Definition A distortion that modifies an association between an exposure and an outcome, caused by the presence of an indication for the exposure. confounding by indication is a bias frequently encountered in observational epidemiologic studies of drug effects. confounding by indication poses a significant threat to the validity of nonexperimental studies assessing effectiveness and safety. this jama guide. Indication Bias Definition.
From criticalthinking.cloud
presentation bias definition Indication Bias Definition confounding by indication poses a significant threat to the validity of nonexperimental studies assessing effectiveness and safety. then, based on earlier work and current project activities, we developed a framework for the assessment of the risk of bias and confounding across a body of observational study. a type of confounding bias that occurs when a symptom or. Indication Bias Definition.
From helpfulprofessor.com
78 Types of Bias (2024) Indication Bias Definition confounding by indication poses a significant threat to the validity of nonexperimental studies assessing effectiveness and safety. this jama guide to statistics and methods reviews the use of methods to control for confounding by indication in clinical studies that assess the potential effect of a. confounding by indication is a bias frequently encountered in observational epidemiologic studies. Indication Bias Definition.
From thinkingispower.com
Guide to the Most Common Cognitive Biases and Heuristics Indication Bias Definition then, based on earlier work and current project activities, we developed a framework for the assessment of the risk of bias and confounding across a body of observational study. this jama guide to statistics and methods reviews the use of methods to control for confounding by indication in clinical studies that assess the potential effect of a. . Indication Bias Definition.
From mavink.com
Different Bias Types Indication Bias Definition then, based on earlier work and current project activities, we developed a framework for the assessment of the risk of bias and confounding across a body of observational study. confounding by indication is a bias frequently encountered in observational epidemiologic studies of drug effects. a type of confounding bias that occurs when a symptom or sign of. Indication Bias Definition.
From mungfali.com
6 Types Of Bias Indication Bias Definition this jama guide to statistics and methods reviews the use of methods to control for confounding by indication in clinical studies that assess the potential effect of a. confounding by indication poses a significant threat to the validity of nonexperimental studies assessing effectiveness and safety. A distortion that modifies an association between an exposure and an outcome, caused. Indication Bias Definition.
From criticalthinking.cloud
presentation bias definition Indication Bias Definition confounding by indication poses a significant threat to the validity of nonexperimental studies assessing effectiveness and safety. confounding by indication is a bias frequently encountered in observational epidemiologic studies of drug effects. then, based on earlier work and current project activities, we developed a framework for the assessment of the risk of bias and confounding across a. Indication Bias Definition.
From exoewimhv.blob.core.windows.net
Examples Of Labeling Bias at Paul McKee blog Indication Bias Definition confounding by indication is a bias frequently encountered in observational epidemiologic studies of drug effects. a type of confounding bias that occurs when a symptom or sign of disease is judged as an indication (or a contraindication) for a given. A distortion that modifies an association between an exposure and an outcome, caused by the presence of an. Indication Bias Definition.
From www.slideteam.net
Indication Bias Prejudice In Powerpoint And Google Slides Cpb Indication Bias Definition confounding by indication is a bias frequently encountered in observational epidemiologic studies of drug effects. then, based on earlier work and current project activities, we developed a framework for the assessment of the risk of bias and confounding across a body of observational study. A distortion that modifies an association between an exposure and an outcome, caused by. Indication Bias Definition.
From helpfulprofessor.com
35 Media Bias Examples for Students (2024) Indication Bias Definition A distortion that modifies an association between an exposure and an outcome, caused by the presence of an indication for the exposure. a type of confounding bias that occurs when a symptom or sign of disease is judged as an indication (or a contraindication) for a given. confounding by indication poses a significant threat to the validity of. Indication Bias Definition.
From criticalthinking.cloud
presentation bias definition Indication Bias Definition this jama guide to statistics and methods reviews the use of methods to control for confounding by indication in clinical studies that assess the potential effect of a. A distortion that modifies an association between an exposure and an outcome, caused by the presence of an indication for the exposure. confounding by indication poses a significant threat to. Indication Bias Definition.
From peakup.edu.vn
What is Bias in Statistics? Its Definition and 10 Types Peakup.edu.vn Indication Bias Definition this jama guide to statistics and methods reviews the use of methods to control for confounding by indication in clinical studies that assess the potential effect of a. confounding by indication is a bias frequently encountered in observational epidemiologic studies of drug effects. confounding by indication poses a significant threat to the validity of nonexperimental studies assessing. Indication Bias Definition.